The European Union has reached a historic agreement on the most important reform of the pharmaceutical market in the last 20 years. The agreement, concluded in the final days of Denmark's rotating presidency of the EU Council, aims to strike a balance between stimulating pharmaceutical innovation—particularly for critical antibiotics and drugs for rare diseases—and accelerating the entry of generic drugs, thus making treatments more accessible in all 27 Member States.
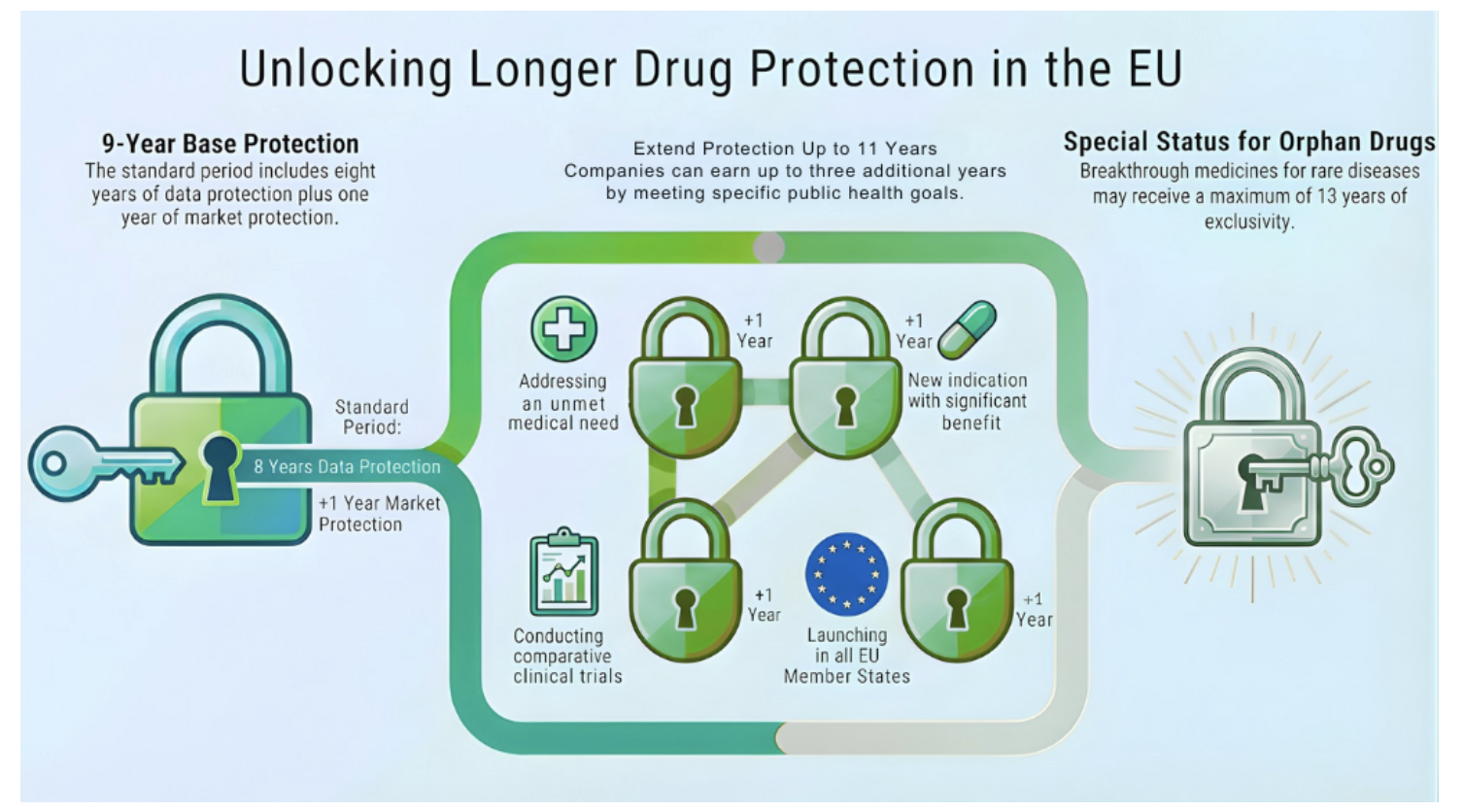
The heart of the reform is the revision of the regulatory data protection and market exclusivity system. The previous period of 10 years is reduced to a base of 9 years, structured as follows:
8 years of data protection: during this period, the results of preclinical and clinical studies remain confidential and cannot be used by generic or biosimilar manufacturers.
1 additional year of market exclusivity: after this period, generic or biosimilar manufacturers can place their products on the market.
This change effectively reduces by one year one of the main protections for innovative drugs, favoring faster access to cheaper versions.
According to Spanish MEP Dolors Montserrat (EPP), one of the main negotiators for the European Parliament, the agreement represents a balanced compromise between the interests of pharmaceutical companies and patients' access to more affordable medicines.
Initially, the European Commission had proposed an even shorter protection period, of 6 years, but the pharmaceutical industry, represented by EFPIA, exerted strong pressure arguing that too short a protection would discourage investment in research and development.
Further details available here: https://healthpolicy-watch.news/eu-clinches-pharma-reform/